![Difference between Strong and Weak Base - with Examples [in Table]](https://d1avenlh0i1xmr.cloudfront.net/0e323ff3-079c-4d5e-b21f-0f4c25082521/differences-between-strong-and-weak-bases-01.jpg)
Difference between Strong and Weak Base - with Examples [in Table]
Strong BaseWeak BaseThey get completely ionized (split up into ions) in water and produce large amounts of hydroxide ions.These only get partially ionized (split up into ions) in water and produce less amount of hydroxide ions.pH value is close to 14 but smaller than it.pH value is closer to 7 but

Examples of Weak Base (5 Examples with Images) - Teachoo Chemistry
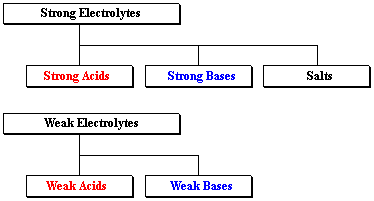
Classifying Electrolytes

Solved [Na+]=1.0M. Table 17.7 lists the common strong bases.
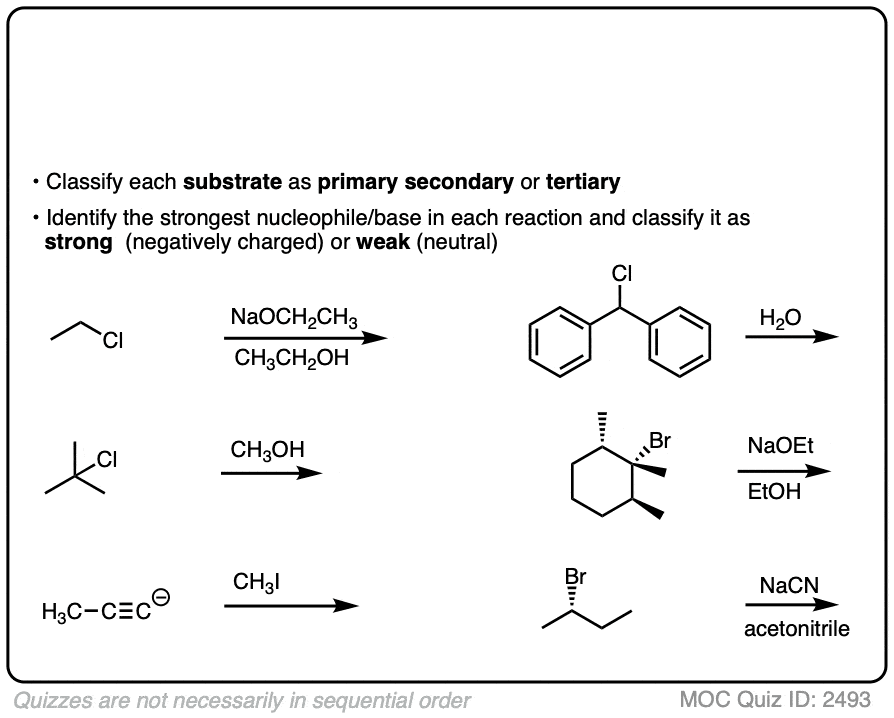
Deciding SN1/SN2/E1/E2 (2) - The Nucleophile/Base

Weak Acid / Strong Base Titration - All pH Calculations
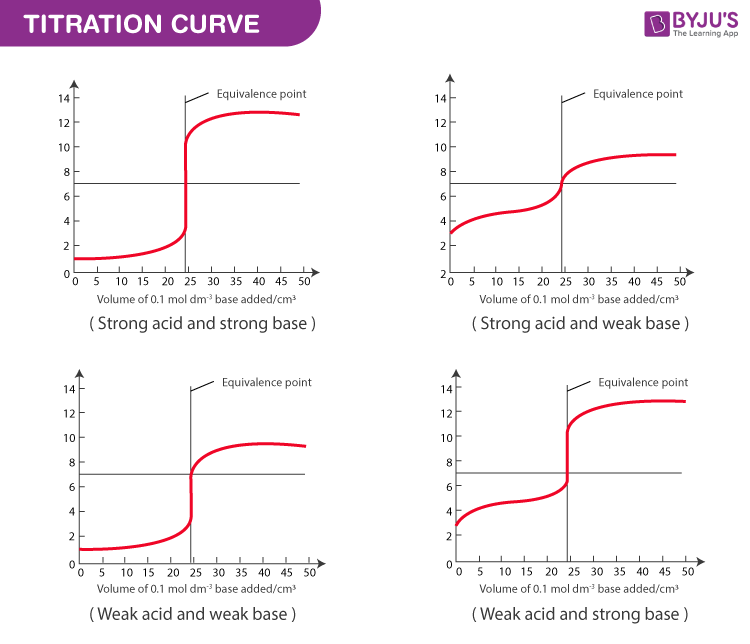
Acid Base Titration - Titration Curves, Equivalence Point & Indicators of Acid Base Titration

Difference Between Strong and Weak Bases Definition, Properties, Reactions, Examples

Weak Acids, Definition, List & Examples - Video & Lesson Transcript
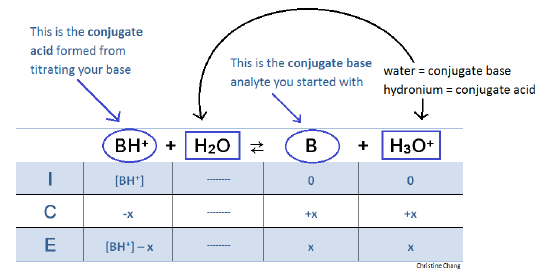
Titration of a Weak Base with a Strong Acid - Chemistry LibreTexts
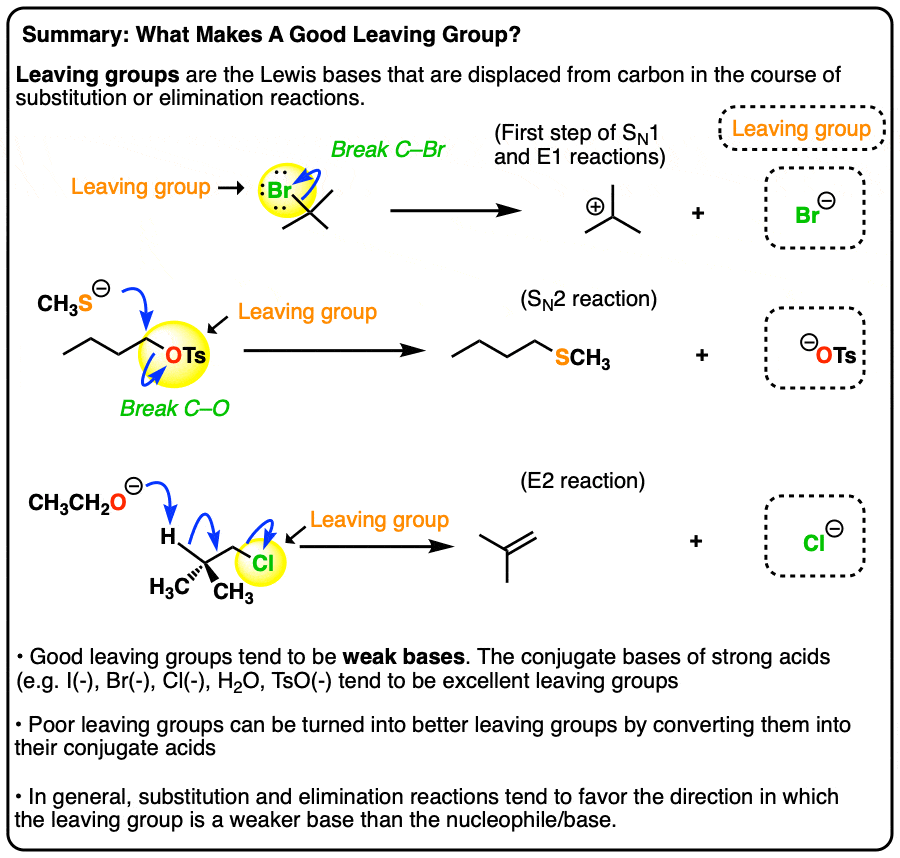
What makes a good leaving group? Master Organic Chemistry

What is different between strong base and weak base? - EduRev Class 10 Question

SN1 SN2 E1 E2 - How to choose the coorect mechanism
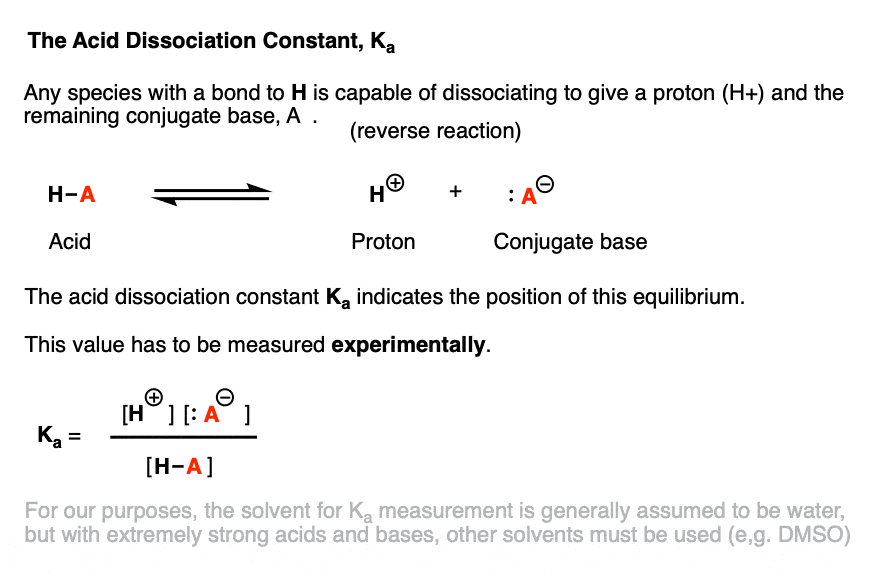
How To Use a pKa Table

How To Memorize The Strong Acids and Strong Bases
Which indicator is used in a strong acid versus a strong base solution? - Quora