
Sanofi Pasteur Announces FDA Approval of Menactra Meningococcal Conjugate Vaccine Indication for Infants
/PRNewswire/ -- Sanofi Pasteur, the vaccines division of the sanofi-aventis Group (EURONEXT: SAN and NYSE: SNY), announced today that the U.S. Food and Drug

Immunogenicity and Safety of a Meningococcal Quadrivalent Conjugate Vaccine in Saudi Arabian Adolescents Previously Vaccinated with One Dose of Bivalent and Quadrivalent Meningococcal Polysaccharide Vaccines: a Phase III, Controlled, Randomized, and
Sanofi Pasteur Starts Shipping New Intradermal Influenza Vaccine With 90 Percent Smaller Needle

MenQuadfi: Package Insert

Rx Item-Menactra Meningitis Vaccine 4Mcg 0.5Ml Vial 5X1 By Sanofi Pasteur

Sanofi Pasteur Announces FDA Approval of Menactra Meningococcal Conjugate Vaccine Indication for Infants
FDA Approves Sanofi Pasteur's Meningococcal Vaccine for Children Aged 9–23 Months

Vaccine History Timeline

An evaluation of emerging vaccines for childhood meningococcal disease, BMC Public Health

Menactra - Meningococcal Vaccine - Clinical Trials Arena

Nt 5 15 5 18 15 final by IAC - Issuu
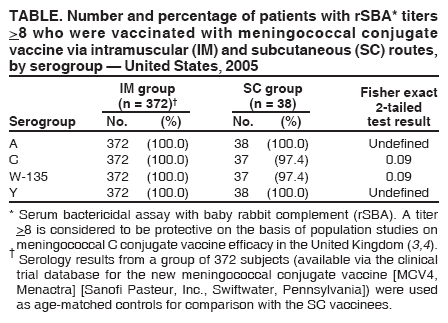
Inadvertent Misadministration of Meningococcal Conjugate Vaccine --- United States, June--August 2005

Synthetic Glycans to Improve Current Glycoconjugate Vaccines and Fight Antimicrobial Resistance

.jpg?rev=cf219796ed7e4c12a8d046d97b29be3d)






