
Update on REMS-Required Testing During COVID-19 Pandemic - MPR
“The completion of some REMS-required laboratory testing or imaging studies may be difficult because patients suspected of having COVID-19 may be self-isolating and/or subject to quarantine,” said FDA Principal Deputy Commissioner Amy Abernethy, MD, PhD.

Post-Thanksgiving COVID-19 bump in Minnesota, but RSV and flu hospitalizations down

REMS Changes Due to the COVID-19 Pandemic

Falsification of at-home isotretinoin pregnancy testing during the COVID-19 pandemic: A case series and proposal of mitigation strategies - ScienceDirect

COVID-19 Resource Center - Texas Hospital Association
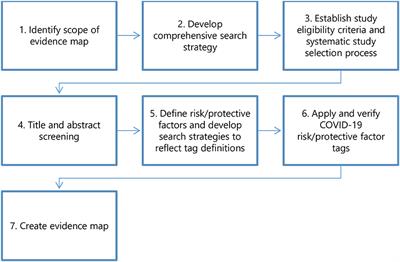
Frontiers Risk and Protective Factors in the COVID-19 Pandemic: A Rapid Evidence Map

Results from a Test-to-Release from Isolation Strategy Among Fully Vaccinated National Football League Players and Staff Members with COVID-19 — United States, December 14–19, 2021

Archived Webinars Readiness and Emergency Management for Schools Technical Assistance Center

COVID-19: Federal Efforts Could Be Strengthened by Timely and Concerted Actions
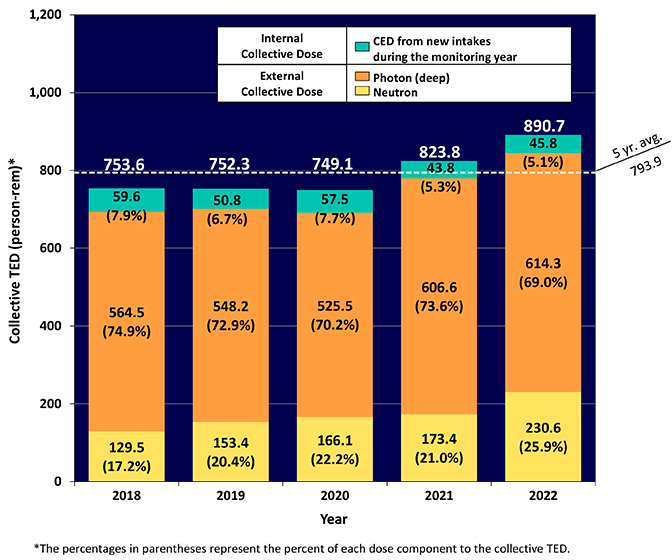
2021 Occupational Radiation Exposure Dashboard

Use of At-Home COVID-19 Tests — United States, August 23, 2021–March 12, 2022
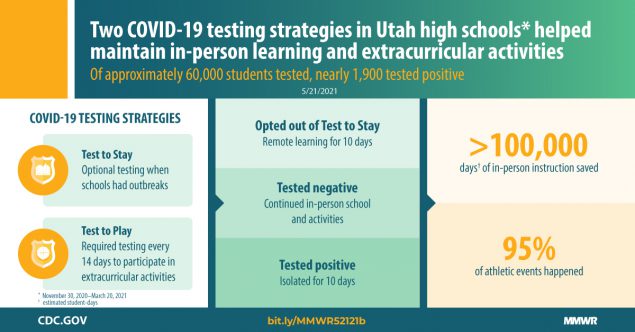
COVID-19 Testing to Sustain In-Person Instruction and Extracurricular Activities in High Schools — Utah, November 2020–March 2021

Post-Thanksgiving COVID-19 bump in Minnesota, but RSV and flu hospitalizations down








