PDF) Comparison of the safety and immunogenicity of an investigational and a licensed quadrivalent meningococcal conjugate vaccine in children 2-10 years of age
PDF | Routine administration of quadrivalent meningococcal conjugate vaccine to adolescents and certain high risk groups is recommended in the United | Find, read and cite all the research you need on ResearchGate

Full article: Immunogenicity and safety of an investigational quadrivalent meningococcal conjugate vaccine administered as a booster dose in children vaccinated against meningococcal disease 3 years earlier as toddlers: A Phase III, open-label
Full article: Meningococcal quadrivalent tetanus toxoid conjugate vaccine (MenACWY-TT, Nimenrix™): A review of its immunogenicity, safety, co-administration, and antibody persistence

Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents.

Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in ≥56-year-olds: A Phase III randomized study - ScienceDirect

PDF) Comparison of the safety and immunogenicity of an investigational and a licensed quadrivalent meningococcal conjugate vaccine in children 2-10 years of age

Full article: Safety and immunogenicity of co-administered meningococcal serogroup B (4CMenB) vaccine: A literature review

Immunogenicity and safety of the quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine (MenACWY-TT) in splenectomized or hyposplenic children and adolescents: Results of a phase III, open, non-randomized study - ScienceDirect

MenACWY-CRM conjugate vaccine booster dose given 4–6 years after priming: Results from a phase IIIb, multicenter, open label study in adolescents and adults - ScienceDirect

Immunogenicity and safety of the quadrivalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine (MenACWY-TT) in 2–10-year-old children: results of an open, randomised, controlled study

Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents.

PDF) Safety and Immunogenicity of Quadrivalent Meningococcal Conjugate Vaccine in 2- to 10-year-old Human Immunodeficiency Virus-infected Children

Immune response, antibody persistence, and safety of a single dose of the quadrivalent meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine in adolescents and adults: results of an open

PDF) Critical appraisal of a quadrivalent CRM197 conjugate vaccine against meningococcal serogroups A, C W-135 and Y (Menveo®) in the context of treatment and prevention of invasive disease
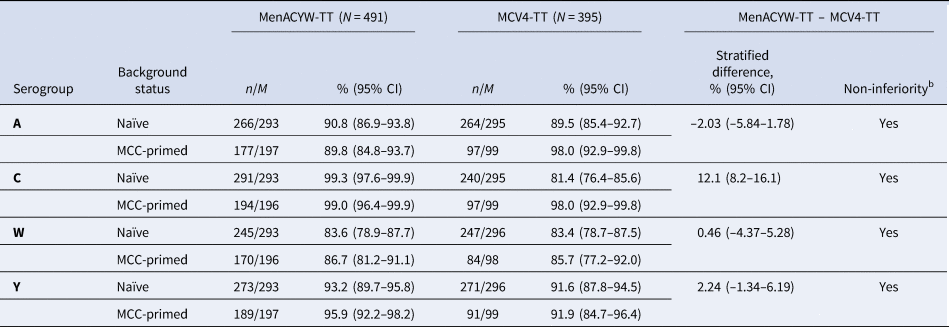
Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) vs. a licensed quadrivalent meningococcal tetanus toxoid-conjugate vaccine in meningococcal vaccine-naïve and meningococcal C conjugate vaccine

Meningococcal Serogroup ACWYX Conjugate Vaccine in Malian Toddlers