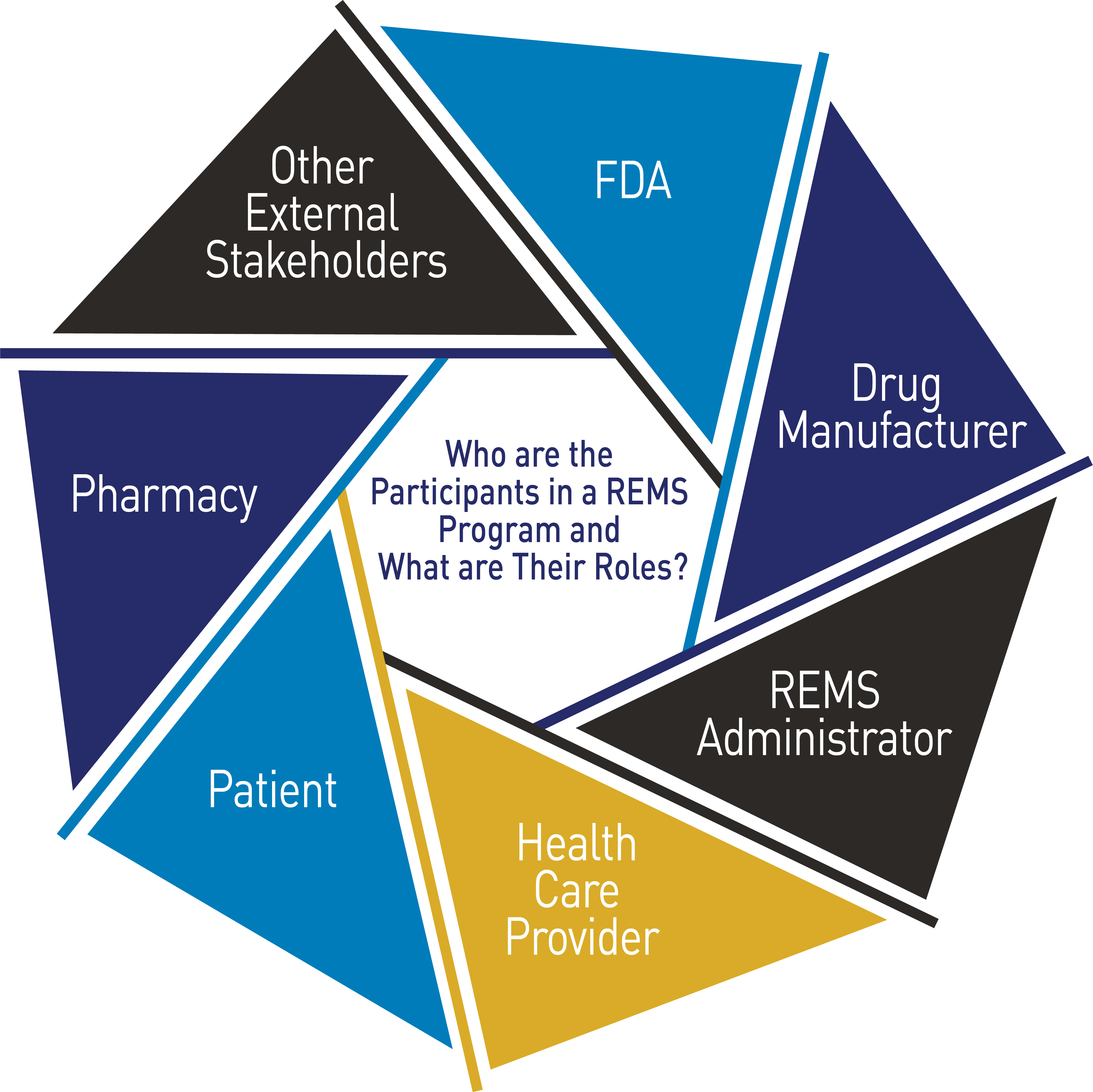
Understanding The FDA's Current Focus On Risk Evaluation And
lt;p>The FDA recently asked for comments about how the government handles vendor change requests from drug sponsors with risk evaluation and mitigation strategies. So, we asked a REMS expert to help us understand why the agency is focusing on the broad-reaching program and what it could mean for drug manufacturers with REMS products in their portfolios.</p>
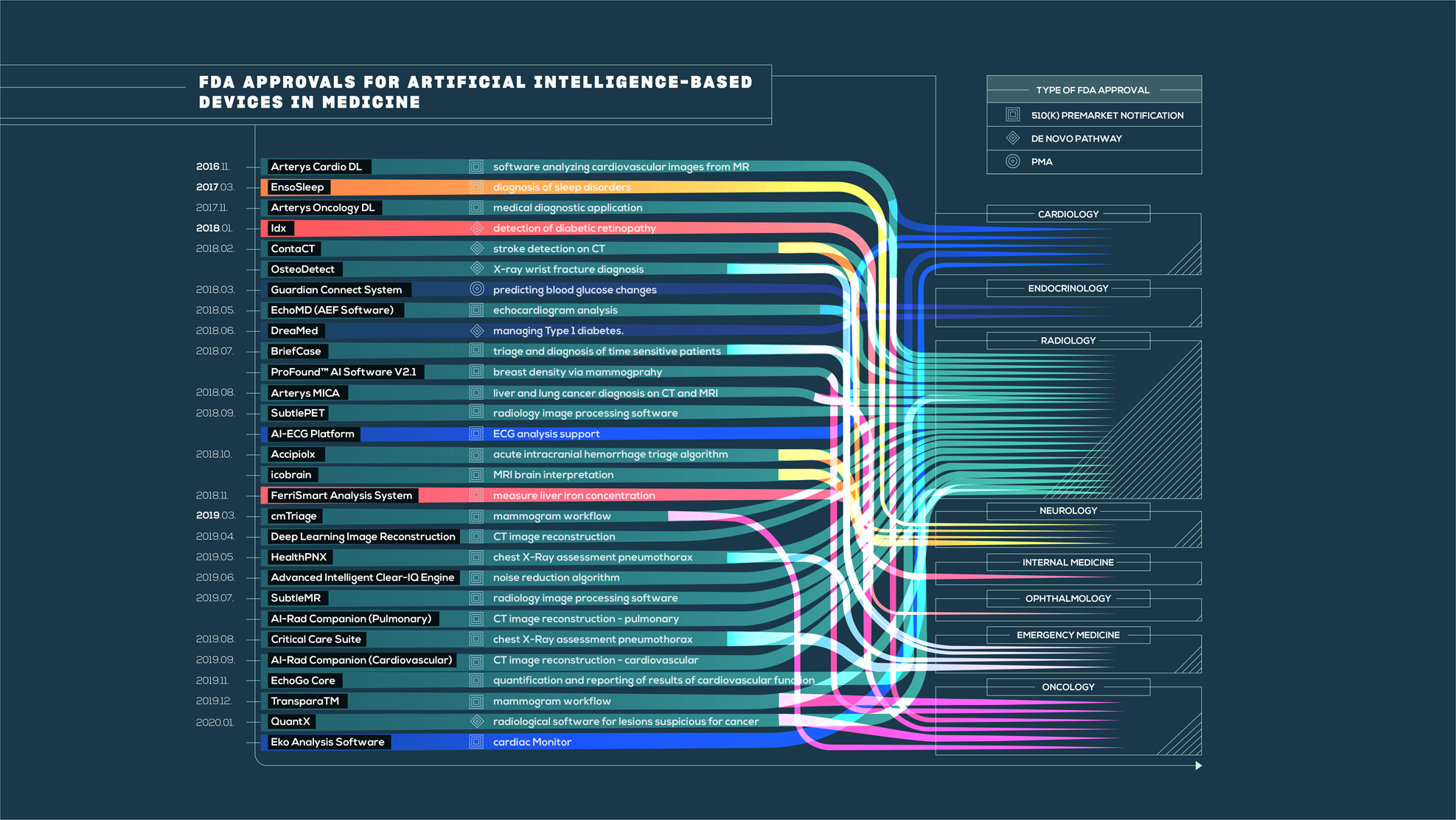
The state of artificial intelligence-based FDA-approved medical

A Decade of FDA-Approved Drugs (2010–2019): Trends and Future
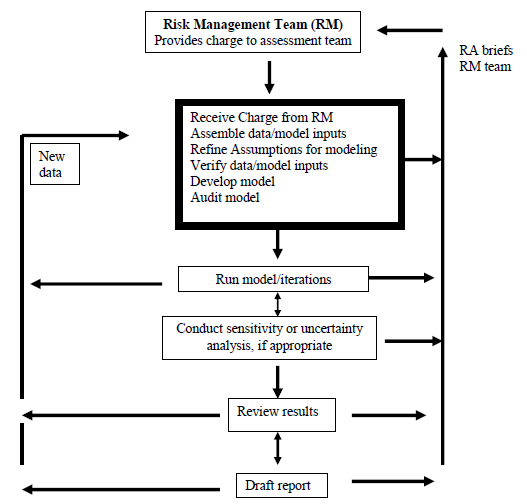
Initiation and Conduct of All Major Risk Assessments within a

Understanding US Food and Drug Administration (FDA) Approval Processes

Conducting Risk-Benefit Assessments and Determining Level of IRB

2021 Dietary Guidance to Improve Cardiovascular Health: A
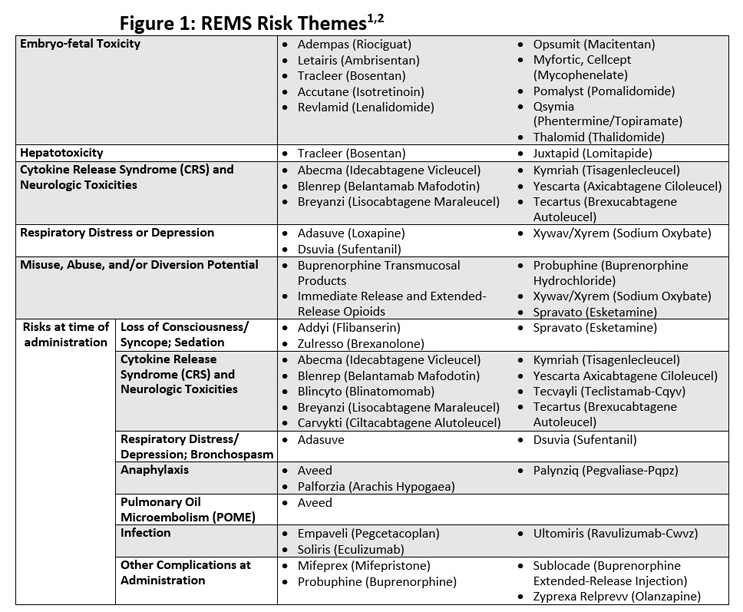
Avoid Launch Delays By Planning For An FDA-Required REMS Risk
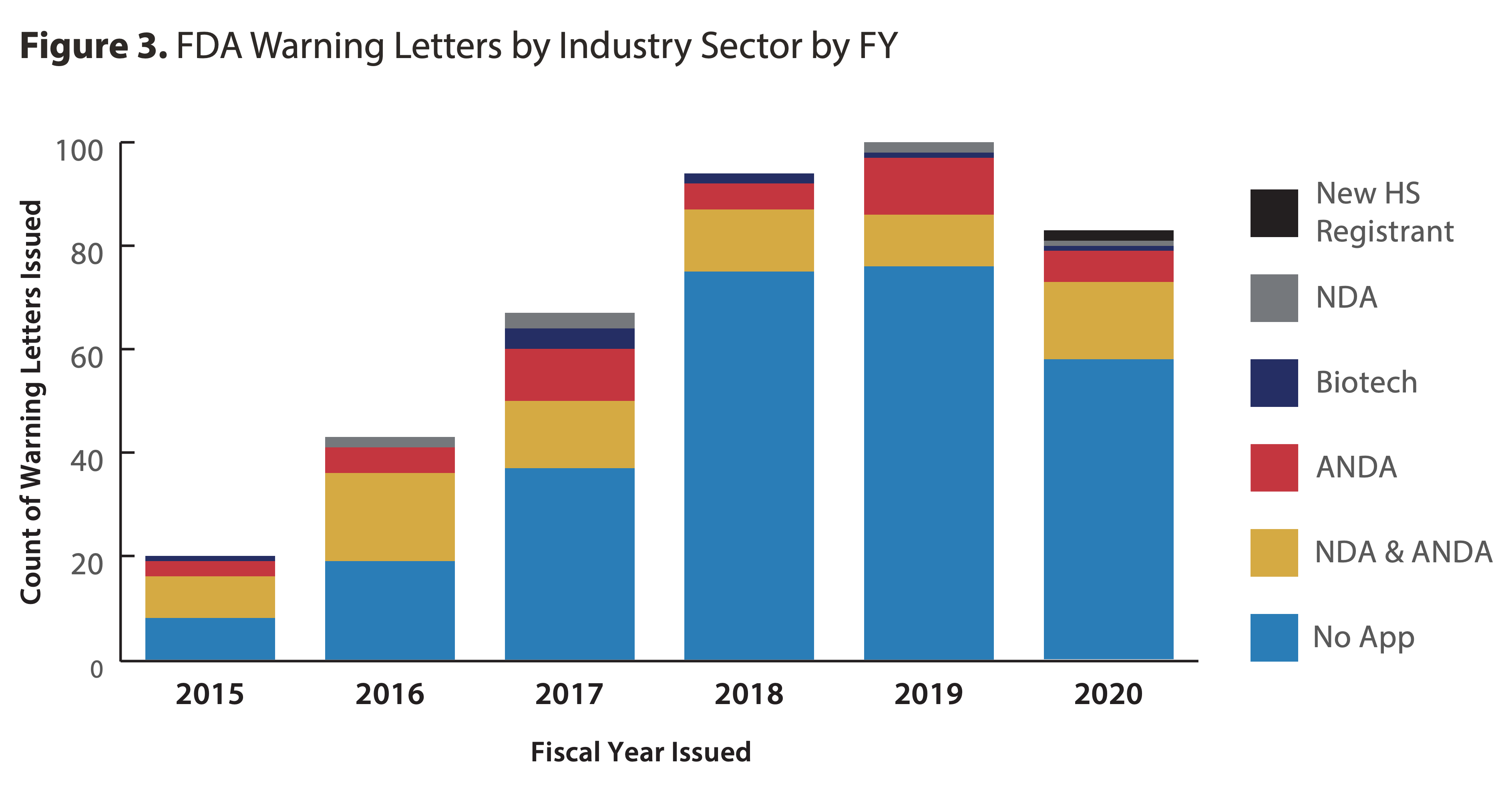
FDA Warning Letter & Inspection Observation Trends [Updated 2023]

What is Risk Management? - Steps in Risk Management System

Emerging Strategies for Drug-Product Comparability and Process
FDA Medical Device Classification: Classes and Examples

US dairy must focus on safety to meet FSMA demands: DeLaval